In this Essay I focus on hydrogen-bonding, that weak bonding which is an essential property of water and biologically important molecules.
Before we start, I want to acknowledge that this will be a very sketchy discussion. To be complete would require a book or two on biochemistry, molecular biology and physiology. A more complete exposition of the chemistry and biochemistry background is given in the “Science Background” section of my web book.
Here are two very general chemical properties that are required to enable living things that metabolize, reproduce, and grow:
- Complexity, to enable living things to carry out many different biological functions; to transmit information within the organism and to descendants;
- “Just Right” Chemical Stability. If biologically important molecules are too stable, metabolism and reproduction will be impossible—species will be inert and necessary chemical reactions won’t occur; if molecules are too reactive, then they won’t be in existence long enough to carry out required chemical function
THE “JUST RIGHT” SOLVENT, WATER
“Water comes down from heaven as rain, and although it is always the same in itself, it produces many different effects, one in the palm tree, another in the vine, and so on throughout the whole of creation. It does not come down, now as one thing, now as another, but while remaining essentially the same, it adapts itself to the needs of every creature that receives it.”
“Office of Readings” (Monday, Week 7 of Easter), from a catechetical instruction by St. Cyril of Jerusalem.
Water is called the “universal solvent” because it dissolves many kinds of molecules, enabling a variety of chemical reactions to occur. One property of water that aids solubility is hydrogen bonding, discussed below. Another is the ability to form loosely bound complexes of water with the components of salts, positive and negative ions, as shown in the illustration below for dissolving salt, NaCl:
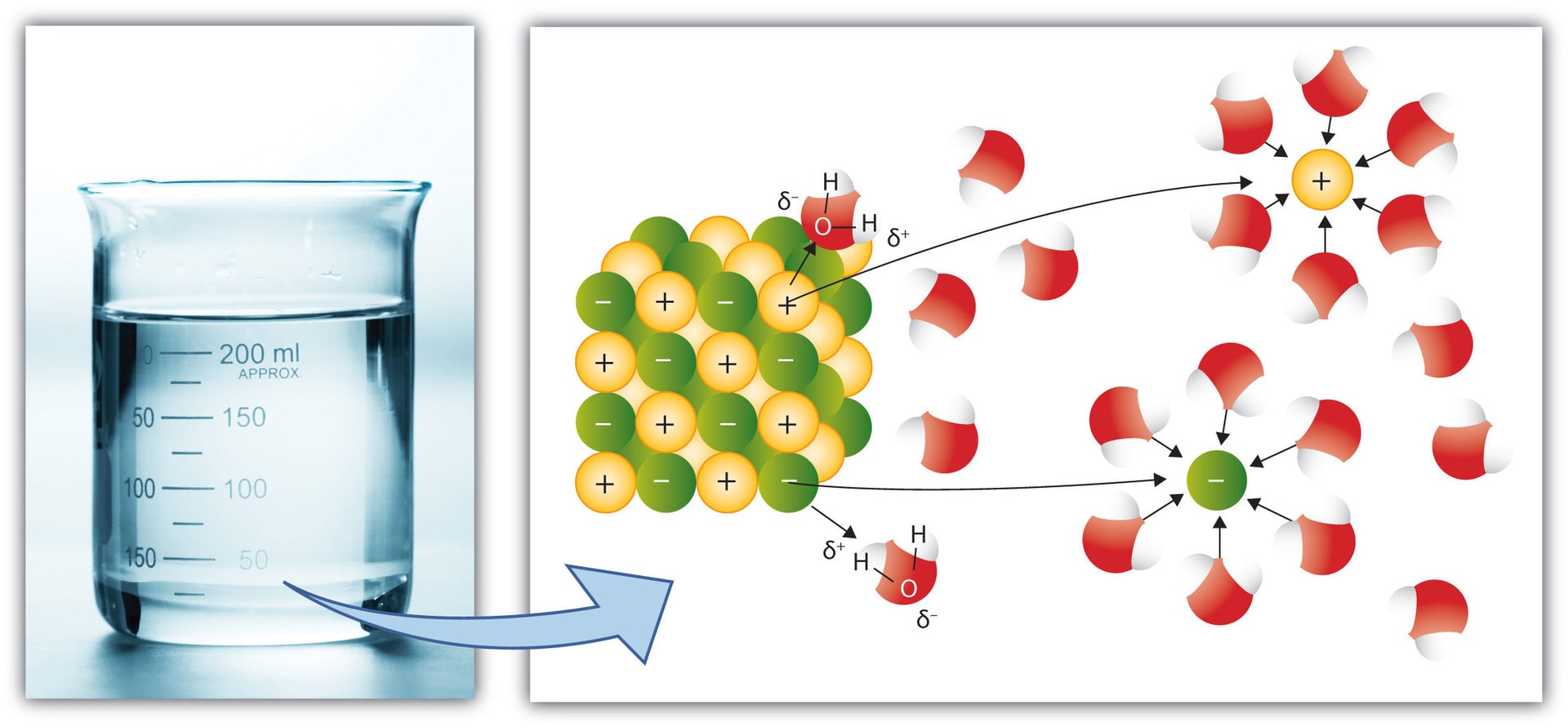 Sodium Chloride (NaCl, salt) dissolving in water (H2O); yellow balls represent Na+ ions with unit positive charge; green balls, Cl- ions with unit negative charge; blue spheres, Hydrogen (H) in H2O; red spheres, oxygen (O) in H2O. Note the small positive charge, d+ on the H atoms in H2O and the small negative charge, d- , on the O atoms. from Wikimedia Commons.
Sodium Chloride (NaCl, salt) dissolving in water (H2O); yellow balls represent Na+ ions with unit positive charge; green balls, Cl- ions with unit negative charge; blue spheres, Hydrogen (H) in H2O; red spheres, oxygen (O) in H2O. Note the small positive charge, d+ on the H atoms in H2O and the small negative charge, d- , on the O atoms. from Wikimedia Commons.
In the diagram above the slightly negative charged oxygen part of H2O is attracted to the positively charged sodium ion, Na+; the slightly positively charged hydrogen part of H2O is attracted to the negatively charged chloride ion, Cl-. Loosely bound complexes of Na+ ion and water molecules and Cl- ion and water molecules are formed to be in solution. The illustration is diagrammatic; sizes of ions and atoms are not to scale. The same type of solvation process applies for many other salts, compounds of potassium, calcium and other metal ions that have roles in biochemistry.
THE “JUST RIGHT” BOND STRENGTH OF THE HYDROGEN BOND: A ZIPPER, NOT GLUE
It has been recognized that hydrogen bonds restrain protein molecules to their native configurations, and I believe...it will be found that the significance of the hydrogen bond for physiology is greater than that of any other single structural feature.
–Linus Pauling, “The Nature of the Chemical Bond”
Let’s imagine God thinking about how He will design nature:
“Now I want chemistry to have not only strong interactions between atoms, but also gentle ones: so that complicated structures can unfold and rewind easily, and so that big and small molecules can come together and join for reactions and go apart readily–Velcro or a zipper, not glue or nails. What should I use? I have it–a hydrogen bond.” (Note: please don’t criticize me for heresy here– I know God holds an infinite number of thoughts and plans simultaneously in His infinite mind.)
Here’s the basic idea: H (hydrogen) bonded to O (oxygen) as in H-O-H (water) shows a slight positive electrical charge; :O, oxygen, with a pair of unbonded (lone) electrons (the two dots in front of the O), shows a slight negative charge. Similarly, :N (nitrogen), with a pair of lone electrons, shows a slight negative charge, and N-H, hydrogen bonded to nitrogen, show a slight positive charge. There is an electrical attraction between these small positive and negative charges; there is also, as nmr experiments have recently shown, a contribution from chemical bonding (sharing of electrons) to hydrogen bonding, so that it is more than simple electrostatic interaction.
In the figure below are shown the types of hydrogen bonds important in molecules of biological interest.
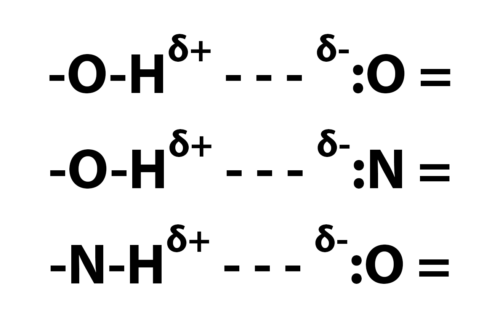 The single dashes represent single bonds; The = signs, double bonds; The – – -, hydrogen bonds; The ” : “, lone pair electrons; Superscripts, delta plus and delta minus, represent small net positive and negative charges.
The single dashes represent single bonds; The = signs, double bonds; The – – -, hydrogen bonds; The ” : “, lone pair electrons; Superscripts, delta plus and delta minus, represent small net positive and negative charges.
Hydrogen bonds energies are about 1/20 to 1/30 the value of ordinary covalent bonds, so the hydrogen bonds can be broken much more easily than covalent bonds; for example the O-H bond energy is about 430 kJoules/mole, whereas the O-H – – – :O hydrogen bond energy is 21 kJoules / mole.
I’ll summarize the role hydrogen bonds play in biochemistry and molecular biology below. A more extended lesson on basic chemistry is given in the “Science Background” section of the web-book.
“JUST RIGHT” BUILDING BLOCKS FOR PROTEINS: AMINO ACIDS
There are 20 amino acids that are the building blocks for proteins. Each amino acid molecule has a COOH, (this acidic carboxyl group releases protons in solution), a NH2, (this basic amine group accepts protons from solution) attached to a central carbon. Also attached to that group are 20 other different chemical parts, symbolized by “R”.
The 20 amino acids that combine to build proteins are shown here. Proteins perform several essential functions:
- catalysis, to speed up biochemical reactions (as enzymes);
- transport of smaller species through the organism and through cells (for example, hemoglobin transporting oxygen);
- building material for tissue;
- engines to move tissue and muscle.
- antibodies to remove unwanted and dangerous stuff
In all these functions, parts of the proteins bind loosely to other parts of the protein and thus form appropriate structures that are essential to their function. This is shown very nicely in the TED YouTube video, by Professor Ken Dill (SUNY/Stony Brook).
https://youtu.be/zm-3kovWpNQ
Another nice YouTube video showing protein flexibility is here. I should emphasize again that the flexibility and ability to form different structures is enabled by hydrogen bonds, bonds that can be zipped and unzipped readily, not glued or nailed. The diagram below illustrates how protein structures change when they act as a catalyst, that is as an enzyme:
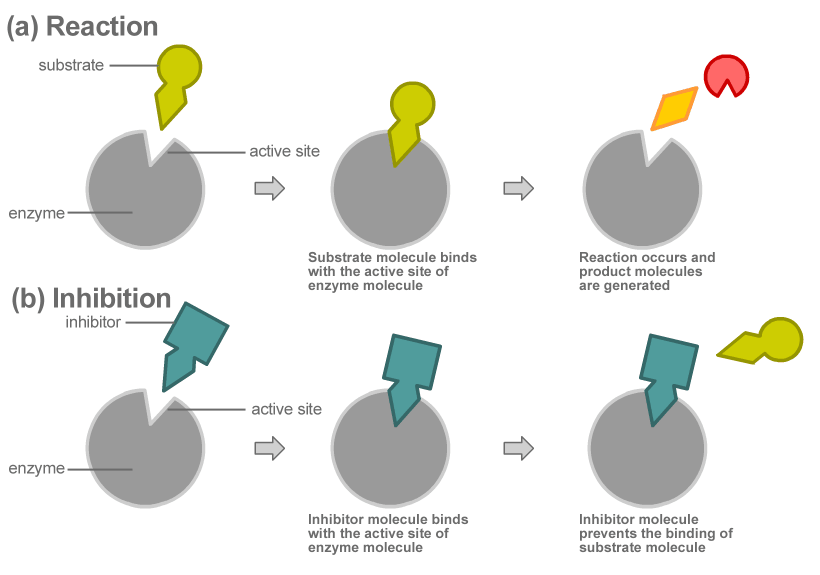 Diagram illustrating how protein structure changes to facilitate chemical reactions from Wikimedia Commons.
Diagram illustrating how protein structure changes to facilitate chemical reactions from Wikimedia Commons.
The hydrogen bond plays an equally necessary and fascinating role in the structure of DNA and the synthesis of proteins that DNA directs.
You can read more here!

